

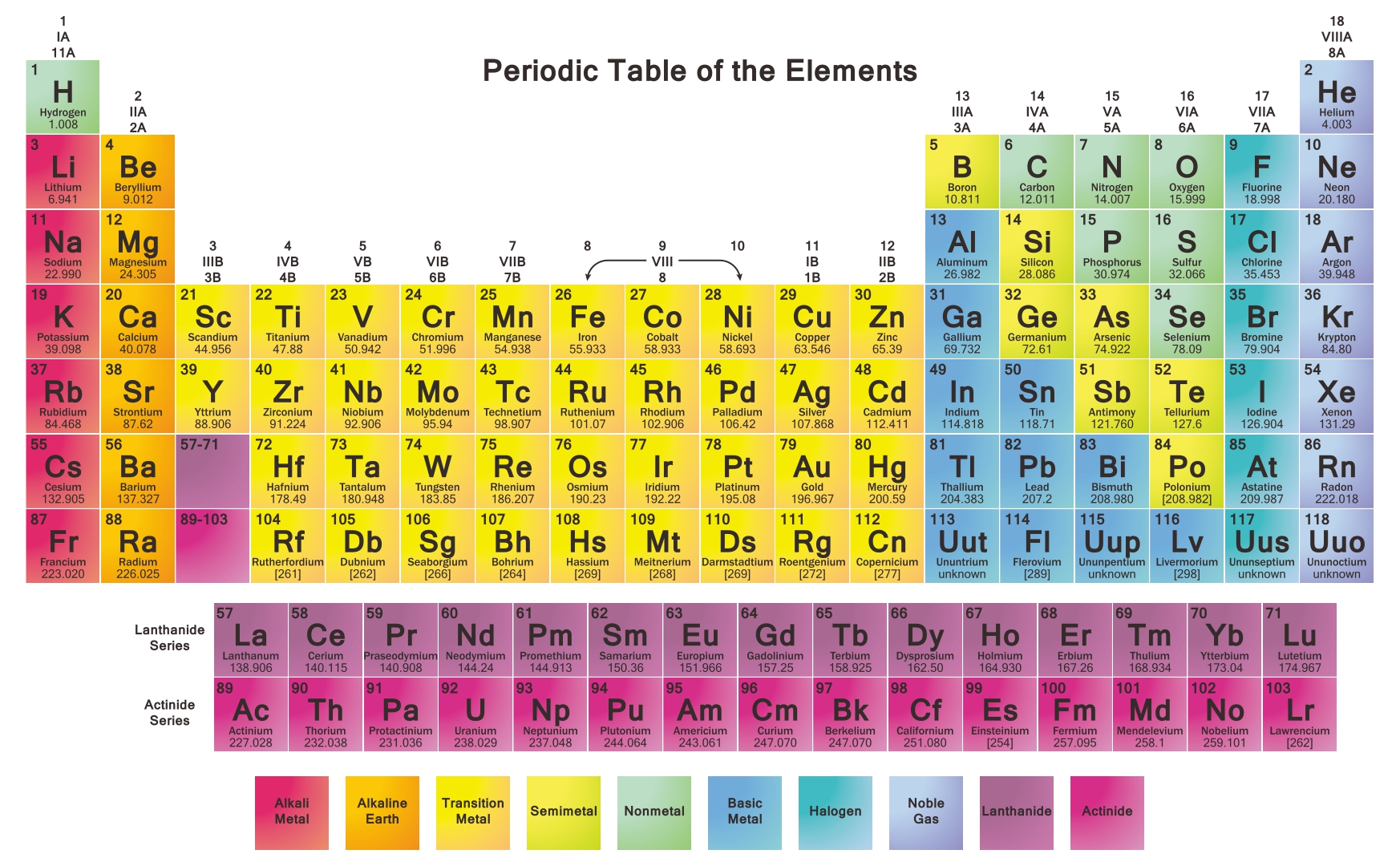
The two rows of elements below the main body of the table (the lanthanides and actinides) are metals. Metals are on the left side of the table. See the study guide on groups in the periodic table for more information. Location of Metals on the Periodic Table Over 75 of the elements are metals, so they fill most of the periodic table.
#Where are metals located on the periodic table full#
The ions formed have a full outer electron shell, so are very stable. Atoms of non-metal elements gain electrons in some of their reactions to form negative ions.Atoms of metal elements give away electrons in their reactions to form positive ions.The reactions of elements are related to the number of electrons in their outer shells: Atoms of group 7 elements have seven electrons in their outer shell, and atoms of group 0 elements, except helium, have eight electrons in their outer shell. Atoms of group 1 elements have one electron in their outer shell, and atoms of group 2 elements have two electrons in their outer shell.Įlements in groups 6, 7 and 0 are non-metals. Atomic structure and the periodic tableĮlements in group 1 and group 2 are metals. Metals are on the left of the periodic table, and non-metals are on the right. The line looks like a series of steps going to the right and down. Additionally, what type of elements form anions Alkali metals and alkaline earth metals always form cations. Elements to the left of this are metals.They are located to the left of the bold line on the right side of the Periodic Table. Unlike metal atoms, nonmetals will gain electrons to become anions. non-metal elements are on the right of the stepped line Also, where are the cations and anions on the periodic table Metal atoms (located on the left side of the periodic table) always lose electrons to become cations.metal elements are on the left of a stepped line starting at B-Al-Si.Metals and non-metals in the periodic table


 0 kommentar(er)
0 kommentar(er)
